Transciption
Current research:
Transcription is the first step in gene expression, where DNA is transcribed into mRNA. We investigate this complex structural and mechanistic process in the eukaryotic and archaeal system. Thereby, we focus on the main enzyme involved in this event, the RNA polymerase (RNAP), and its several transciption factors to gain structural, dynamic and kinetic information by using single-molecule fluorescence microscopy, fluorescence correlation spectroscopy and optical tweezers.
Published projects:
→ Complete architecture of the archaeal RNA polymerase open complex from smFRET and NPS
The molecular architecture of RNAP II-like transcription initiation complexes remains opaque due to its conformational flexibility and size. Here we report the three-dimensional architecture of the complete open complex (OC) composed of the promoter DNA, TATA box-binding protein (TBP), transcription factor B (TFB), transcription factor E (TFE) and the 12-subunit RNA polymerase (RNAP) from Methanocaldococcus jannaschii. By combining single-molecule Förster resonance energy transfer and the Bayesian parameter estimation-based Nano-Positioning System analysis, we model the entire archaeal OC, which elucidates the path of the non-template DNA (ntDNA) strand and interaction sites of the transcription factors with the RNAP. Compared with models of the eukaryotic OC, the TATA DNA region with TBP and TFB is positioned closer to the surface of the RNAP, likely providing the mechanism by which DNA melting can occur in a minimal factor configuration, without the dedicated translocase/helicase encoding factor TFIIH.
DOI: 10.1038/ncomms7161
→ Dynamic Architecture of a Minimal RNA Polymerase II Open Promoter Complex
The open promoter complex (OC) is a central intermediate during transcription initiation that contains a DNA bubble. Here, we employ singlemolecule Fo¨ rster resonance energy transfer experiments and Nano-Positioning System analysis to determine the three-dimensional architecture of a minimal OC consisting of promoter DNA, including a TATA box and an 11-nucleotide mismatched region around the transcription start site, TATA box-binding protein (TBP), RNA polymerase (Pol) II, and general transcription factor (TF)IIB and TFIIF. In this minimal OC, TATA-DNA and TBP reside above the Pol II cleft between clamp and protrusion domains. Downstream DNA is dynamically loaded into and unloaded from the Pol II cleft at a timescale of seconds. The TFIIB core domain is displaced from the Pol II wall, where it is located in the closed promoter complex. These results reveal large overall structural changes during the initiation-elongation transition, which are apparently accommodated by the intrinsic flexibility of TFIIB.
DOI: 10.1016/j.molcel.2012.02.008
→ The Initiation Factor TFE and the Elongation Factor Spt4/5 Compete for the RNAP Clamp during Transcription Initiation and Elongation
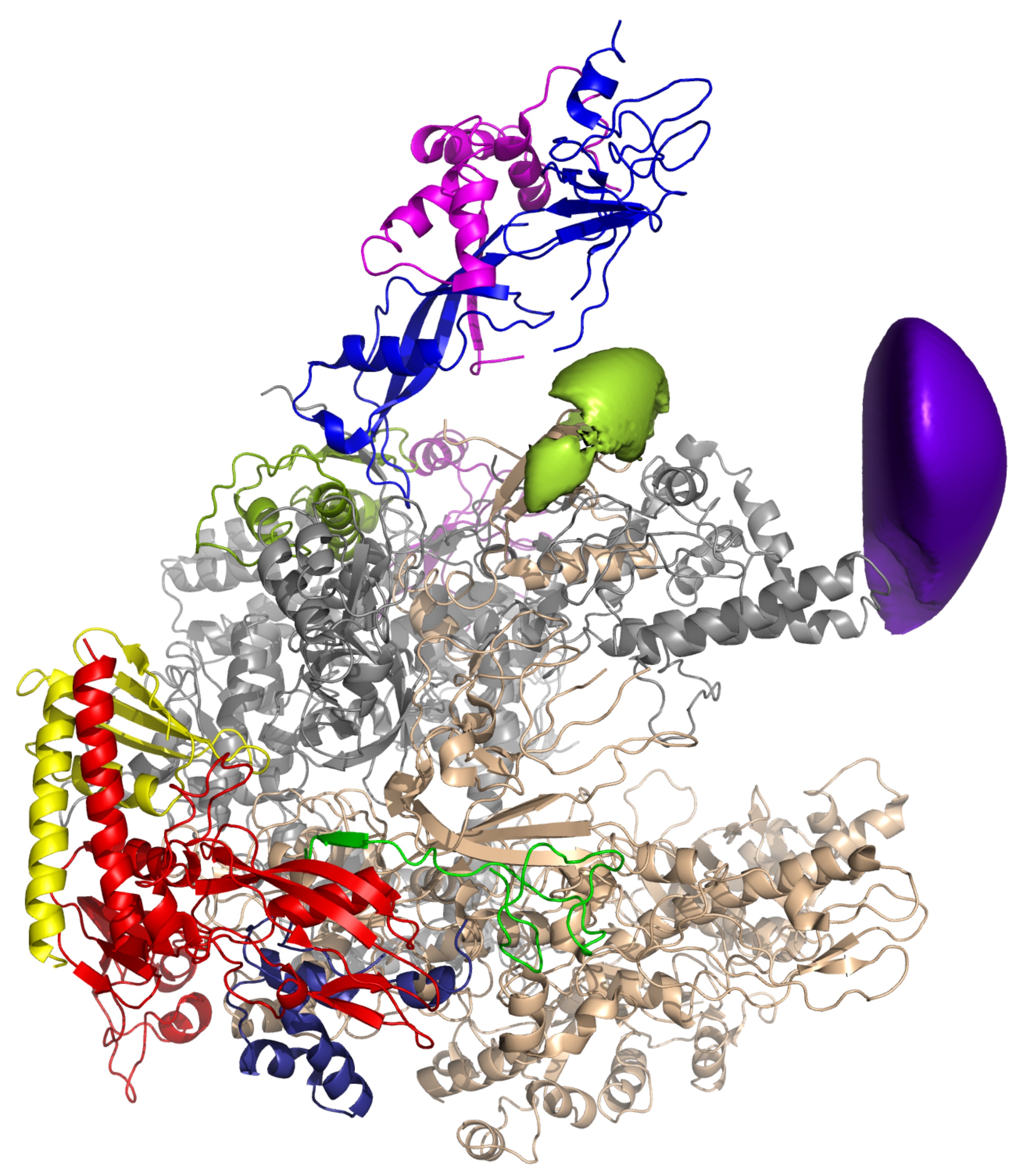
TFIIE and the archaeal homolog TFE enhance DNA strand separation of eukaryotic RNAPII and the archaeal RNAP during transcription initiation by an unknown mechanism. We have developed a fluorescently labeled recombinant M. jannaschii RNAP system to probe the archaeal transcription initiation complex, consisting of promoter DNA, TBP, TFB, TFE, and RNAP. We have localized the position of the TFE winged helix (WH) and Zinc ribbon (ZR) domains on the RNAP using single-molecule FRET. The interaction sites of the TFE WH domain and the transcription elongation factor Spt4/5 overlap, and both factors compete for RNAP binding. Binding of Spt4/5 to RNAP represses promoter-directed transcription in the absence of TFE, which alleviates this effect by displacing Spt4/5 from RNAP. During elongation, Spt4/5 can displace TFE from the RNAP elongation complex and stimulate processivity. Our results identify the RNAP ‘‘clamp’’ region as a regulatory hot spot for both transcription initiation and transcription elongation.
Molecular Cell 43 263-274, July 22,2011
