Weidinger lab - research
All animals have evolved strategies to deal with damage due to injury or disease, but the ability to regenerate lost or damaged organs and appendages varies greatly in different species. Unfortunately, humans and other mammals are quite poor at regenerating, while other vertebrates, like salamanders and fish, can efficiently re-grow lost limbs/fins and regenerate many internal organs including their hearts. Currently it is a mystery why mammals can’t do what these lower vertebrates achieve. We hope that elucidating the mechanisms that zebrafish use to regulate regeneration will one day result in therapies aimed at activating regenerative potential in human organs.
Mechanisms of zebrafish fin and bone regeneration
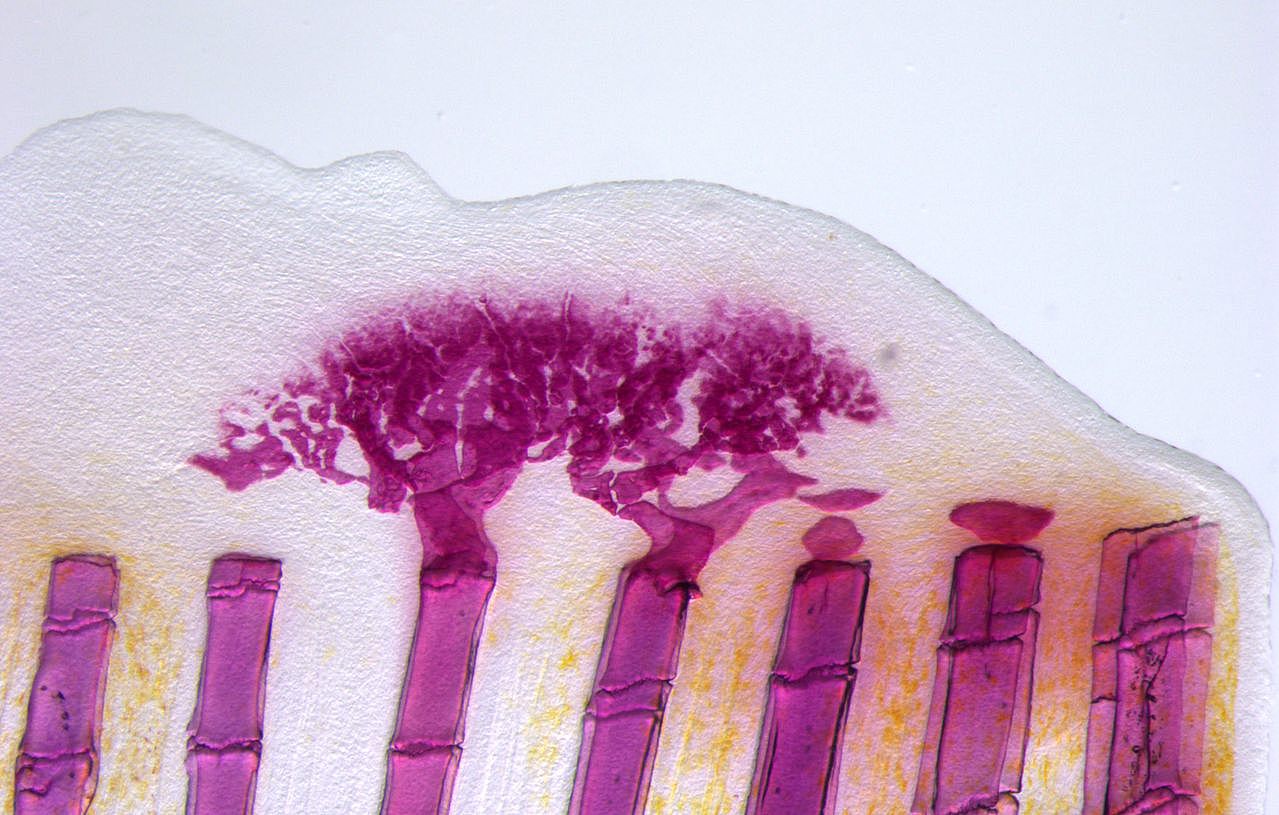
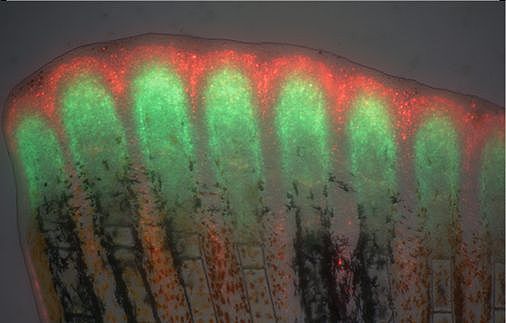
Unlike mammals, zebrafish can completely and repeatedly regenerate lost appendages, that is their fins. This occurs via formation of a population of progenitor cells, the blastema, which contains the precursors of the regenerating tissue. How the blastema forms is a central question in regeneration research. Intriguingly, we have found that mature osteoblasts dedifferentiate and give rise to blastema cells after fin injury. The fact that differentiated cells, and not stem cells, represent important source cells for tissue regeneration has recently been recognized also in several other systems. Currently, we aim to elucidate the molecular mechanisms regulating this fascinating process of cellular plasticity.
In addition, we are interested in understanding the molecular pathways regulating regenerative growth and patterning. We have found that Wnt/beta-catenin signaling is required for formation and proliferation of the blastema; intriguingly however, it acts largely indirectly and orchestrates regenerative growth via secondary signals. Currently, we aim to elucidate how Wnt signaling sets up organizing centers within the blastema.
Selected first or communicating author publications of our group related to fin & bone regeneration:
Sehring et al. 2022, elife
Mishra et al. 2020, Developmental Cell
Owlarn et al. 2017, Nature Communications
Sehring & Weidinger 2016, Current Opinion in Genetics & Development
Wehner et al. 2015, JoVE
Wehner et al. 2015, Trends in Genetics
Geurtzen et al 2015, Development
Wehner et al. 2014, Cell Reports
Grotek et al. 2013, Development
Azevedo et al. 2011, PLOS One
Knopf et al. 2011, Developmental Cell
Stoick-Cooper et al. 2007, Genes & Development
Stoick-Cooper et al. 2007, Development

Our work on bone regeneration is embedded in the Collaborative Research Center (SFB) 1149: "Danger Response, Disturbance Factors and Regenerative Potential after Acute Trauma".
Mechanisms of zebrafish heart regeneration
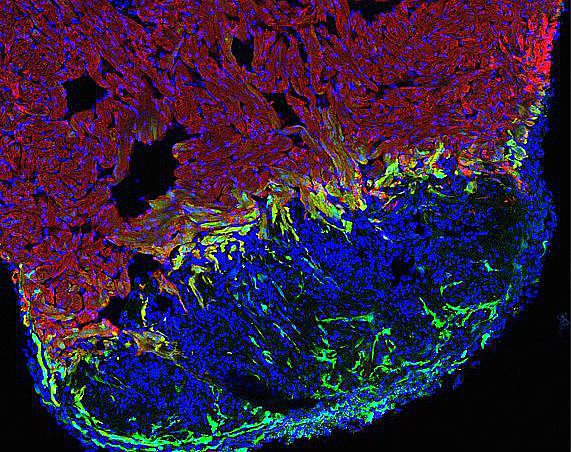
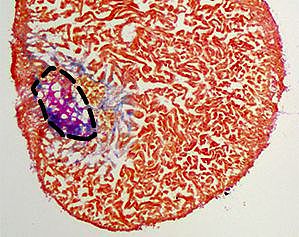
Heart damage, usually caused by infarction, is a leading cause of death in humans. After cardiomyocyte loss the damaged part of the myocardium in humans undergoes extensive scarring and fibrosis, and the cardiomyocytes lost to injury cannot be replaced. Thus, infarction results in permanent damage to the heart. In contrast, zebrafish are able to regenerate their hearts after injury without permanent scarring. Importantly, cardiomyocytes efficiently proliferate to restore to lost muscle tissue. We have found that injuries causing necrotic death of the myocardium, which are similar to human heart infarction, are efficiently regenerated.
Regeneration involves the proliferation of differentiated cardiomyocytes and the coordinated growth of myocardium, endocardium and epicardium. Currently, the molecular mechanisms regulating these processes are only beginning to emerge. We found that BMP signaling is essential for heart regeneration. Interestingly, it promotes cardiomyocyte proliferation during regeneration, but is not required for physiological cardiomyocyte proliferation. In addition, BMP signaling has also been reported to be active in mammalian hearts after injury, but there it plays a detrimental role, promoting cardiomyocyte apoptosis. Thus, our findings indicate that the differential capacity of zebrafish and mammals to regenerate the heart depends, at least in part, on different responses of cardiomyocytes to the same conserved signaling pathway, namely BMP signaling. We currently aim to elucidate how BMP signaling promotes regeneration in zebrafish and why it does not in mammals.
Selected first or last author publications of our group related to heart regeneration:
Bertozzi et al. 2022, Dev. Biol.
Bertozzi et al. 2021, Dev. Biol.
Wu et al. 2016, Developmental Cell
Wu and Weidinger 2014, Curr Pathobiol. Reports
Schnabel et al. 2011, PLOS One
Knopf et al. 2010, PNAS
Nemtsas et al. 2010, J Mol Cell Card
Ueno et al. 2007, PNAS

Our work on heart regeneration is embedded in the Collaborative Research Center (SFB) 1506: "Aging at Interfaces".

