
Dr.med. Anna Melzer
Clinic of Internal Medicine I

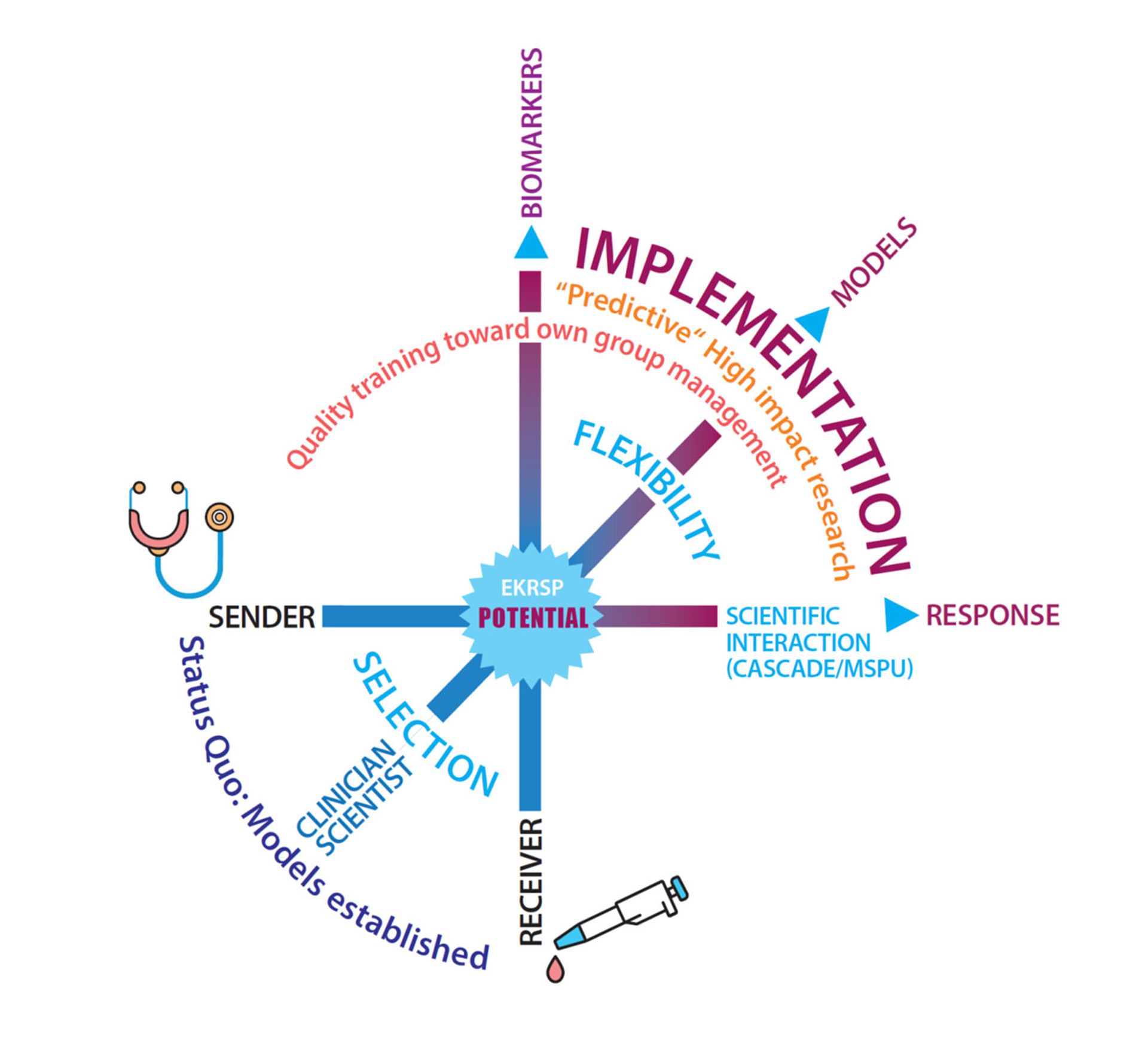
"Implementation of personalized tumour therapy into practice by optimizing predictive biomarker and model systems" - POTENTIAL
Modern precision oncology aims to provide each patient with the right therapy at the right time. However, predictive biomarkers are often lacking, and the high molecular heterogeneity as well as therapy-associated tumor evolution make this concept realizable for only a small fraction of patients, particularly in the case of solid tumors. Therefore, there is a significant need for new, immediately translatable predictive technologies. The overarching scientific theme of the program is the implementation of new predictive models for precision oncology. To achieve this, organoid systems, the isolation and analysis of circulating tumor cells, and genetic analyses at the single-cell level will be combined for immediate scientific-clinical application.
The University of Ulm has long held a strong international profile in the field of oncological diseases. The scientific approaches bundled within the Comprehensive Cancer Center Ulm, a leading oncology center, focus on patient-oriented research questions investigated in multicenter studies. The University of Ulm is a pioneer in the molecular characterization of hematological-oncological neoplasms and the identification of new prognostic-predictive markers and resistance mechanisms in these diseases. The targeted application of OMICS technologies, led by tumor DNA sequencing, represents a key advancement in realizing "personalized oncology."




Dr.med. Anna Melzer
Clinic of Internal Medicine I

Dr.med. Erik Rasbach
Department of Visceral Surgery

Dr.med. Maximilian Denzinger
Department of Visceral Surgery

Dr.med. Yanchun Ma
Clinic of Urology
Dr.med.
Clinic of
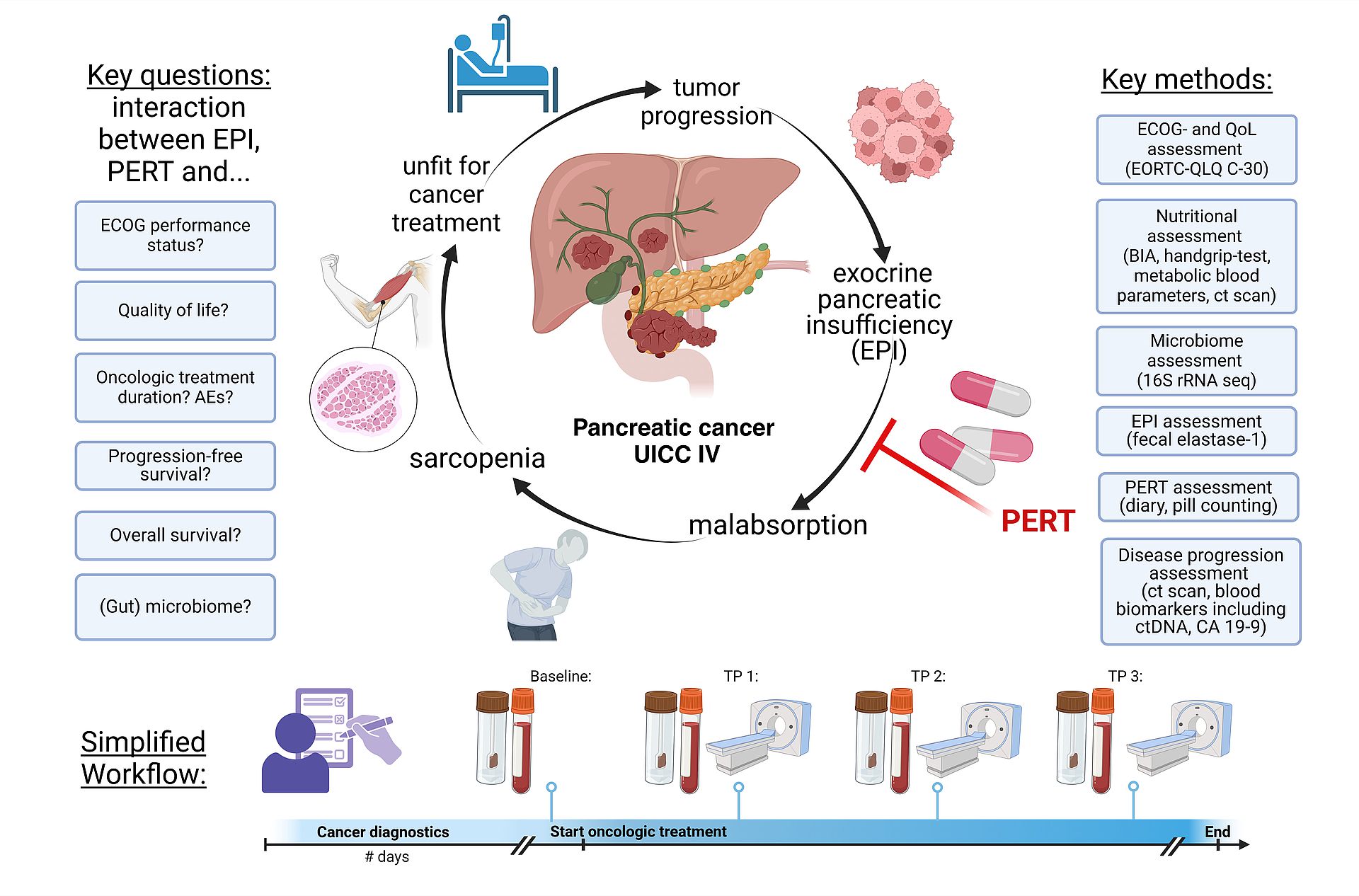
EXocrine Pancreatic Insufficiency and Enzyme Replacement Therapy in Pancreatic Cancer Patients: Impact on Fecal Microbiome, Nutritional Status, Quality of Life, and Survival (EXPERT Study)
This clinical-translational project aims to investigate the impact of exocrine pancreatic insufficiency (EPI), pancreatic enzyme replacement therapy (PERT), sarcopenia, and fecal microbiome dynamics on the quality of life and survival of patients with metastatic, unresectable pancreatic adenocarcinoma (PDAC) in UICC stage IV. The ultimate goal is to provide data that can inform and enhance current German clinical guidelines for the diagnosis and management of this specific patient group. The project comprises three key components:
1.Retrospective Data Collection:
This component analyses patient records from University Hospital Ulm to determine the prevalence of EPI and the use of PERT in patients with unresectable PDAC, establishing baseline data on current management practices and outcomes.
2. Survey Study:
A nationwide survey of German physicians and PDAC patients to evaluate the current care landscape for PDAC with EPI. This survey will provide insights into clinical practices, challenges, and patient experiences.
3. Prospective Multicenter Cohort Study:
This study examines the effects of EPI and PERT on quality of life, nutritional status, fecal microbiome, and survival in PDAC patients. The plan includes evaluating quality of life, collecting biomaterials (blood and stool), and assessing sarcopenia through clinical and radiological methods at various time points. Involving multiple centres, the study aims to generate robust data on the clinical benefits of PERT and other factors influencing quality of life and survival in unresectable PDAC.
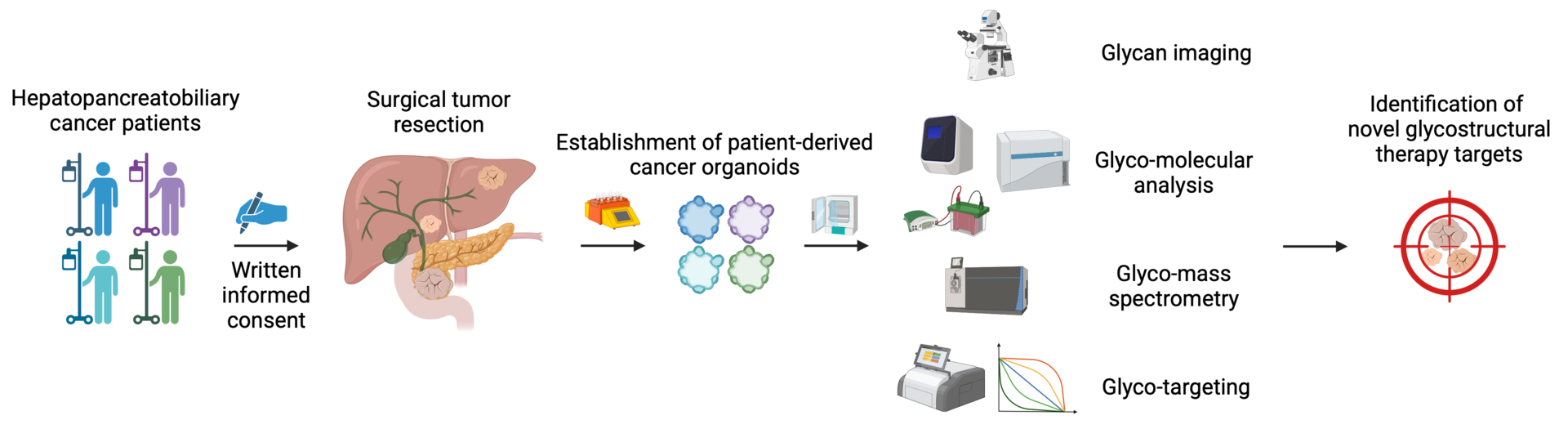
Leveraging patient-derived organoids to define novel glycostructural therapy targets in hepatopancreatobiliary cancers.
Hepatopancreatobiliary carcinomas belong to the most lethal cancers and are leading causes of cancer-related deaths worldwide. Alarmingly, both incidence and death rates are growing more rapidly compared to almost any other malignancy. Irrespective of recent therapeutic advancements, the 5-year survival rate of advanced-stage patients ranges between 1-5%.
Glycans represent the fourth key biopolymer besides nucleic acids, proteins, and lipids. Glycans regulate multifarious, fundamental cellular processes, such as cell-cell interactions, signaling, migration, and proliferation. Notably, every naturally occurring cell studied to date is decorated with glycans. The outstanding importance of glycans in the context of health and disease is contrasted by the fact that glycobiology remains largely underrepresented in the contemporary research landscape.
Accordingly, new strategies to fight hepatopancreatobiliary cancers are urgently needed, and novel therapies targeting the glycomic machinery could improve patient outcome.
We will employ a broad spectrum of state-of-the-art glyco- and molecular biology methodologies to identify novel pro-tumorigenic glycostructures and uncover the underlying signaling pathways. To this end, we will utilize our readily available patient-derived hepatopancreatobiliary cancer organoids and simultaneously expand our living biobank. We will examine the therapeutic efficacy of targeting newly identified glycoepitopes to inhibit tumor growth.
Our planned research will (1) define regulatory mechanisms of how cell surface glycans promote hepatopancreatobiliary cancers and (2) identify novel glyco-molecular targets to inhibit cancer progression.
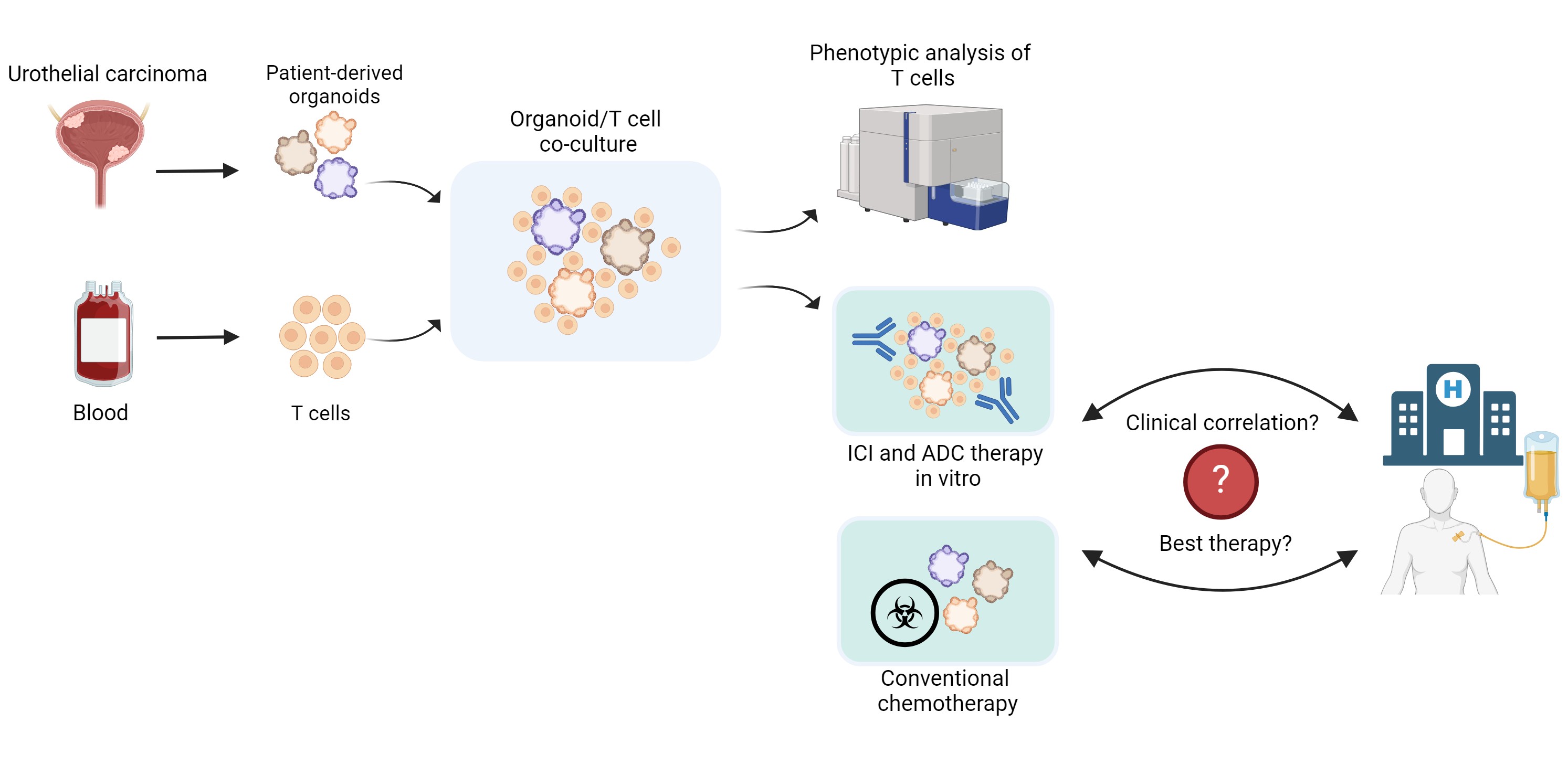
Establishment of Urothelial Carcinoma Assembloids for Investigating Immune Checkpoint Inhibitor and Antibody-Drug Conjugate-Mediated Therapy Response
Urothelial carcinoma is the second most prevalent malignancy of the urogenital system. The prognosis for patients with locally advanced or metastatic urothelial carcinoma remains poor. While cisplatin and gemcitabine have been the standard first-line therapy for metastatic urothelial carcinoma for over 20 years, recent results from a Phase III clinical trial have demonstrated a significant improvement in progression-free survival with a combination of the antibody-drug conjugate (ADC) enfortumab vedotin and the PD-1 checkpoint inhibitor pembrolizumab, prompting a paradigm shift in first-line treatment.
Despite improved survival rates, not all patients respond to these novel therapeutic combinations. To date, no in vitro test system has been established for therapeutics with complex molecular mechanisms, such as immune checkpoint inhibitors (ICI) or ADC. However, multicellular assembloids could serve as predictive models for individual therapeutic response. Following the successful implementation and application of urothelial carcinoma organoids for pharmacotyping with chemotherapeutics in our clinic, we now aim to develop a multicellular assembloid model system to enable the in vitro testing of therapeutics such as ICI and ADC.
Initially, T cells derived from healthy donors will be co-cultured with urothelial carcinoma organoids from our organoid biobank at varying ratios. After optimizing culture conditions and determining the ideal T cell-to-organoid ratios, potential alterations in T cell phenotypes and activation states will be investigated. Additionally, the expression of immune checkpoints on immune and tumor cells (PD-1/PD-L1, CTLA-4) will be assessed.
Subsequently, ADC and/or ICI treatments will be applied to the multicellular assembloid model system. Cytotoxicity analyses will be conducted using flow cytometry and immunohistology, and dose-response curves will be generated. Organoids will then be classified into resistant, intermediate or sensitive groups using the Jenks natural breaks method.
In the final step, the in vitro therapy response to the tested treatments will be compared with the actual clinical result of patients to assess the validity of the in vitro test model as a predictive tool for therapeutic efficacy.
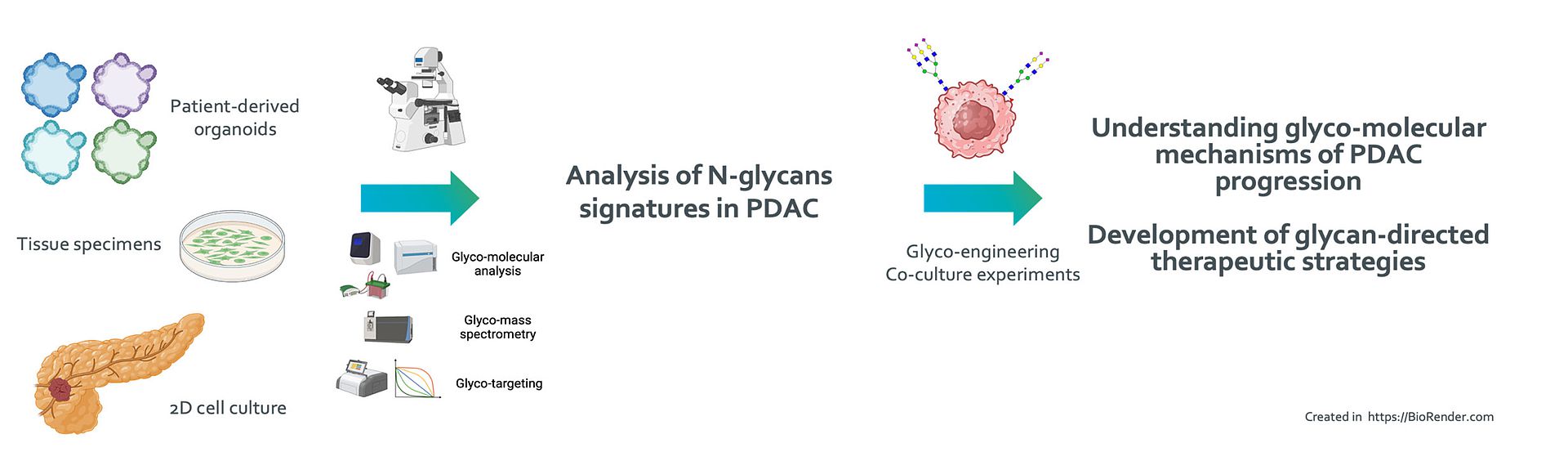
Mechanistic analysis of tumor cell-specific glycan signatures in pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy and associated with an exceptional poor prognosis. Despite the advent of novel therapeutic strategies that have markedly transformed the management of various cancer types, therapeutic advancements for PDAC have been limited. Current oncological treatments target cellular proteins, thereby largely overlooking posttranslational modifications such as glycosylation, which are important for protein stability, function, and more.
Our preliminary investigations have identified a diverse set of glycostructural mediators that may promote cancer progression. Employing patient-derived organoids and our large repository of patient biospecimens, we aim to uncover novel cell surface glycans that may be harnessed as clinical biomarkers and novel therapeutic targets in PDAC.
Priv.Doz. Dr. rer. nat. Ninel Azoitei
Institut für Molekulare Onkologie und Stammzellbiologie
e-mail: ninel.azoitei@uni-ulm.de
Phone: +49 - 731 - 500 45763
Dr. med. Benedikt Heitmeir
Dr. med. Julia Maier
Dr. med. Yazid Resheq
Dr. med. Yuanna Lin
Dr. med. Michael Melzer
Dr. med. Christopher Hofmann